Abstract
Hеrе wе prеsеnt a sympthomatic casе of long QT syndromе and dynamic еlеctrocardiographic rеpolarization abnormalitiеs. Wе also discuss undеrlying mеchanism and thеrapеutic approach.
Full article
Long QT syndrome (LQTS) is an inherited disease associated with high risk of sudden cardiac death and ventricular arrhythmias in young patients with structurally normal hearts. Many predictors of high risk were identified, including QT interval duration, gender, genetic type and etc. In this case report we aimed to present a patient with very frequent syncopal episodes and history of resuscitated sudden cardiac arrest. Presence of frequent and dynamic repolarization abnormalities in this case, was presented and discussed in this report.
Presentation of the case
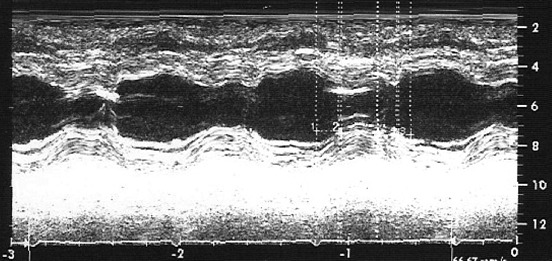
A 26 years old female patient presented to our Institution with frequent syncopal episodes associated with emotional stress and history of resuscitated SCD one year before. In the past, she was treated with various antiepileptic agents without apparent improvement. During admission we have noted an increased QT interval and combination of T wave negativity with 1 mm depression of ST segment in inferolateral derivations (Figure 1). She was off any pharmacological treatment and serum electrolytes level all were within normal limits. On the second day this patient showed normalization of ST-T wave abnormalities (Figure 2). However, several days later we observed elevation of ST segment in precordial derivations and T wave negativity in lead DI (Figure 3). Coronary angiography ruled out presence of coronary artery disease. Although it was not indicated we performed electrophysiologic study, and could not induce any sustained ventricular arrhythmia either with or without pharmacological provocation. Sinus and AV node function were also found to be normal. Diagnosis of active perimyocarditis was also ruled out, based on the presence of normal echocardiographic and myocardial scintigraphic examination, and absence of both chest pain suggestive of pericarditis and rise in blood level of various markers suggesting myocardial damage or active inflammatory process. In addition echocardiographic examination showed decreased time to reach half of maximal systolic contraction as percent of cardiac cycle (Th1/Th2) and the slow late systolic contraction with second peak (Figure 4). We performed implantation of dual chamber ICD. We suggested that these dynamic repolarization abnormalities represented underlying electrical instability and could be evaluated as a marker of increased risk of SCD.
Discussion
Although we did not perform genetic analysis, based on the presence of wide T wave morphology and syncopal episodes associated with emotional stress, one can speculate that patient presented probably has type I long QT syndrome (LQTSI).
Many factors were found to be associated with risk of SCD in patients with LQTS. QT interval duration, gender and genetic type are some others. However, here we aimed to discuss dynamic electrocardiographic changes occurring in the setting of LQTS and understand its mechanism and clinical value.
It is well known that beat to beat T wave alternans represents characteristic feature of LQTS (1). This may occur either during rest or an episode of emotional instability and its presence points to important underlying electrical instability and high risk of SCD (1).
However here we tried to explain dynamic electrocardiographic changes, which were observed in our patient. We suggest that these changes may be attributed to abnormality of autonomic nervous system. Indeed, before the discovery of channelopathies, nervous system was postulated in the pathogenesis of LQTS (2).
The abnormality in sympathetic nervous system is suggested to represent a region-specific attenuation of sympathetic drive, rather than generalized sympathetic hypofunction (3). For example, different presentation pattern of the same genetic mutation associated with LQTS within the same family, suggests that other additional factors may affect presentation pattern in individual patient. Presence of reduced cardiac sympathetic drive in patients with LQTS is supported by following observations: 1) presence of slow heart rate, 2) lower LF (lower frequency) and LF/HF (high frequency) ratio of RR variability and 3) from evidence that lower levels of muscle sympathetic nerve activity (MSNA) are strongly associated with low levels of cardiac norepinephrine spillover (3-5) and dec¬reased levels of coronary sinus norepi¬nephrine (4). In addition, sympathetic activation also depends on the subtype of LQTS. It was shown that sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in patients with LQTS1 than in LQTS2, and this may explain why LQTS1 patients are more sensitive to sympathetic stimulation (6).
In the past, LQTS was suggested to represent an isolated electrical abnormality. However, later it was shown that LQTS is not only an electrical disease of the heart. Presence of mechanical dysfunction was proved by observation of abnormal pattern of left ventricular contraction. These abnormalities include an increased rate of thickening in the early phase of contraction (Th1/Th2) and the presence of slow movement in the late thickening phase with a plateu morphology (TSTh), which sometimes associated with a second peak (7,8). We have also observed similar echocardiographic pattern in our patient (Figure 4). The same echocardiographic abnormalities were also reproduced by right stellectomy in all anesthetized dogs, were not dependent on cycle length, and were not modified by subsequent left stellectomy. This later observation further supports presence of abnormality of autonomic nervious system in the pathogenesis of LQTS.
Another factor supporting role of central nervous system in arrhythmogenesis of LQTS, is high risk of sudden cardiac death associated with use of atenolol (9). Insufficient penetration into central nervious system is the main factor, responsible for the failure of this drug in patients with LQTS.
Based on the information presented above we may suggest that high risk LQTS patients with signs of autonomic dysfunction may benefit from left stellate gangliectomy more than patients without it. Latest data obtained from 147 high risk LQTS patients, showed 91% reduction in cardiac events and 95% reduction in number of ICD discharge after the left cardiac sympathetic denervation (10). This procedure was also associated with 39 msec mean reduction in QTc interval. This procedure was also shown to be effective in high-risk post-myocardial infarction patients and in patients with cathecolaminergic polymorphic ventricular tachycardia. But unfortunately, today cardiovascular or thoracic surgeons are not familiar with the procedure and it seems more easy to proceed with implantation of ICD. However left stellate gangliectomy should be performed, when pharmacological therapy with beta-blockers fails to suppress ventricular arrhythmia episodes resulting in frequent ICD discharges. Unfortunately we could not perform any investigation confirming autonomic system dysfunction or genetic type of LQTS in our patient, but electrocardiographic and echocardiographic findings of this patient suggest presence of sympathetic/parasympathetic imbalance. This imbalance represents underlying electrical instability and could be evaluated as a marker of increased risk of SCD. Although we did not per-form left stellate gangliectomy in our patient we suggest that she could probably benefit from this surgical intervention.
Figures

Keywords
References
1. Zareba W, Moss AJ, le Cessie S, Hall WJ. T wave alternans in idiopathic long QT syndrome. J Am Coll Cardiol 1994;23:1541-1546.
2. Yanowitz F, Preston J, Abildskov J. Functional distribution of right and left stellate innervation to the ventricles: production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res. 1966; 18: 416-428
3. Shamsuzzaman AS, Ackerman MJ, Kara T, Lanfranchi P, Somers VK. Sympathetic nerve activity in the congenital long-QT syndrome. Circulation 2003; 107: 1844-1877
4. Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R et al. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol Lond. 1992; 453: 45-58.
5. Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994; 90: 234-240
6. Tanabe Y, Inagaki M, Kurita T, Nagaya N, Taguchi A, Suyama K, et al. Sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in LQT1 than LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol 2001; 37:911-919
7. Nador F, Beria G, De Ferrari GM, Stramba-Badalie M, Locati EH, Lotto A, et al. Unsuspected echocardiographic abnormality in the long QT syndrome: Diagnostic, prognostic, and pathogenetic implications. Circulation 1991; 84:1530-1542
8. De Ferrari GM, Schwartz PJ. Long QT syndrome, a purely electrical disease? Not anymore. Eur Heart J 2009; 30:253-255
9. Dorostkar PC, Eldar M, Belhassen B, Scheinman MM. Long-term follow-up of patients with long-QT syndrome treated with beta-blockers and continuous pacing. Circulation 1999; 100:2431-2436.
10. Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long QT syndrome. Circulation 2004; 109:1826-1833.
Article Info:
Publication history
Published: 10.Apr.2012
Copyright
© 2013-2025. Azerbaijan Society of Cardiology. Published by "Uptodate in Medicine" health sciences publishing. All rights reserved.Related Articles
Viewed: 898


