Abstrakt
Məqsəd: Azərbaycan əhalisində perindopril ilə müqayisədə valsartanın antihipertenziv müalicə effektivliyinin gender fərqlərini qiymətləndirmək olmuşdur Material və metodlar. Paralel qruplar arasında açıq-çarpaz nəzarət (crossover ) öyrənilməsi 3 ay ərzində aparılıb. 45-65 yaş arasında olan ümumi 120 xəstələr hər qrupa 30 adam daxil olan dörd qrupa bölündü: 1A, 1B kişi daxil və 2A, 2B - qadın.İlk altı həftə 1A üçün 2 A qrup xəstələr 1B isə, 2 B alt xəstələr gündə valsartan 80-160 mq (Diovan) ilə müalicə olunmuşdur, gündə perindopril 5-10 mq (Prestarium) qəbul etmişdir. Sonra terapiya çapraz dəyişdirilib. Nəticələr. İlkin müayinə zamanı kişilər və qadınlar arasında qan təzyiqi əhəmiyyətli dərəcədə fərqlənməyib. Qadınlarda valsartan fonunda perindoprilə nisbətən sistolik qan təzyiqi kişilərlə müqayisədə daha çox azalmışdır ( р = 0,02 Δ% 16,0 ± 3,2% vs 19,1 ± 4,6%) . Kişilərdə isə perindopril və valsartanın təyini zamanı arterial təzyiqin azalmasında əhəmiyyətli fərqlər aşkar olunmamışdır (Δ% 15,1 ± 14,6 ± 4,5%vs3,4%р=0,54). Nəticə. Valsartan qadınlara srart terapiya kimi perindoprillə müqayisədə nisbətən daha effektiv edir. Kişilərdə perindopril və valsartan ilə start terapiyada effektivəhəmiyyətli fərqlər aşkar etməyib.
Əsas mətn
According to the latest data more than 20 million people have heart failure worldwide. The prevalence and incidence of heart failure are increasing, mostly because of increasing life span, but also because of increased prevalence of risk factors (hypertension, diabetes, dyslipidemia, and obesity) and improved survival rates from other types of cardiovascular disease (myocardial infarction, valvular disease, and arrhythmias [1,2].
In 2011, non-hypertensive congestive heart failure was one of the ten most expensive conditions seen during inpatient hospitalizations in the U.S., with aggregate inpatient hospital costs of more than $10.5 billion [3].
Controversy exists in the medical literature on the difference in treatment of diverse cardiac diseases, including heart failure (HF), between men and women. Some studies showing differences in treatment received by men compared with women [4] affirmed that the latter received angiotensin converting enzyme inhibitors (ACEI) less frequently than men [5,6], underwent fewer diagnostic tests [7,8] and adherence to treatment guidelines was less satisfactory [6] It has been suggested that the differences are due to sex [9], although some works attribute, them to specialty of the physician since, according to them, more women are seen by general practitioners and more men by cardiologists. Others deny the differences, or attribute them to the differential characteristics of the syndrome in women [10-13].
According to J.Ghali’s (Wayne state Unoversity, USA) data presented at the 11-th scientific conference of American society of heart failure in Washington (2007), among 20000 patients, enrolled in the analysis mortality rate was significantly lower in female patients taking angiotewnsin II receptor blockers (ARB) as compared to that in female patients receiving ACE inhibitors (ACEi) which are considered to be the standard of chronic heart failure (CHF) treatment in hypertensive patients [14] .Taking into account these data one can suppose probability of sex-related differences in antihypertensive effect of ARB and ACEi as well.
Drug pharmacokinetics and pharmacodynamics may vary in different ethnic groups [15-16]. In view of the multiethnic composition of Azerbaijan, the purpose of this study was to collect data pertinent to our own local population in terms of comparing the efficacy and tolerability of valsartan (DIOVAN®) an AII antagonist against perindopril (PRESTARIUM®) an angiotensin converting enzyme inhibitor (ACEI) in patients with hypertension and HF.
Material and methods.
The study was conducted in the Scientific Research Institute (SRI) of Cardiology of Azerbaijan named after Dzh. Abdullayev. This was an open- label, parallel group, comparative trial comparing the efficacy of valsartan (80-160 mg) and perindopril (5-10) mg given once daily. Concomitant medication was similar for the duration of the study.
Prior approval of the National Ethics Committee was obtained and all patients gave their written consent to participate in the study. The study was conducted in conformance to good clinical practice guidelines.
A total of 153 patients were initially included to the study. However, 33 patients were not randomized due to multiple reasons such as DBP<95 mm.Hg, abnormal biochemistry, withdrawal of consent, or loss to follow up.
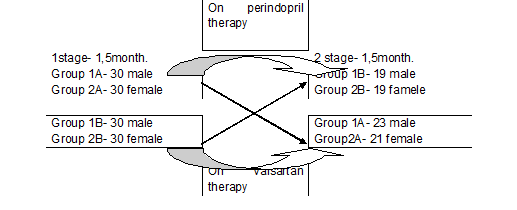
A total of 120 patients of 45-65 years old during 3- month study were split into four groups with 30 people in each: 1A,1B included men, and 2A,2B – women. For the first six weeks 1A, 2A group patiens received perindopril 5-10 mg ( Prestarium) per day,while 1B,2B subgroup patients were treated with valsartan 80-160 mg ( Novartis) per day. Then the therapy was crosswise changed. The study design is presented in fiqure 1.
Figure 1. Scheme crossover trial in parallel groups
Statistical analysis was made using software packages SPSS 13.0. Data are shown as mean values and standart deviation ar as median interquartile range, or as percentage ratio. Nonparametric and parametris criteria for independent samples were used. Distinctions were considered significant at p<0,05.
Results
Table 1 presents clinical chatacteristics of the examined groups. The groups were comparable for age, hypertension duration and degree, systolic BP (BPs) level, diastolic BP (BPd) level, HR, body mass index (BMI).
A total of 120 patients aged 20-45 years with hypertension of 1-2 degrees were examined for 3 months. During the first stage of the study (6 weeks) treatment of one patient discontinued from the group 1B (the reason - adverse event of cough). In 2 stage of the study from group 1A discontinued treatment 23% (n = 7/30) of the patient, from the group 2A - 30% (n = 9/30), from the group 1B - 31% (n = 9 / 29), and because of coughs - 3% (n = 1/29). From 2 B group due to the refusal to continue the treatment 27% (n = 8/30), and because of cough - 10% (n = 3/30) of patients excluded from the study.
Table1. Clinical Characteristics of the groups
Note:1 А – group of men received perindopril; 2А –group of women received perindopril; 1B – group of men received valsartan; 2B – group of women received valsartan.
Finally, were analyzed 82 patients whocompleted thescheduledtreatmentwithin 3 months: ingroup1A-23 patientsingroup 2A-21 ingroup1B- 19 ingroup 2B-19. Thelower bloodpressure (BP) to the target( 140/80mm Hg) all patients.
The analyses of efficacy was based on intent to treat all randomized patients who had baseline and at least one post baseline BP measurement.
At the end ofonephase of the study all patients reducedblood pressure (BP) to the target( 140/90mm Hg). Side effects such asdry coughduring the studywhile taking perindoprilwas recordedin 2 (3.3%) of the 60 menand 3(5%) of the 60 women, as evidenced by the literature, that thecoughoccurswhenan ACEI more oftenin women thanin men.
Both valsartan and perindopril reduced DBP compared to the baseline at all points measured, with similar reduction in two groups. At the beginnigs of the valsartan decreased BPs in women more prominently than perindopril did (∆%19,1±4,6% vs 16,0±3,2%; р=0,02),at that BPd reductions did not distinguish significantly (∆%15,9±9,2% vs 15,2±5,3%; р=0,3).
Men did not demonstrate significant differences in BPs and BPd dynamics in valsartan and perindopril start therapy (∆%15,1±3,4% vs 14,6±4,5%; р=0,54), and (∆%15,3±4,6% vs 16,0±2,2%; р=0,59, respectively).
Discussion
This study has demonstrated that valsartan and perindopril are equally effective in lowering blood pressure in patients with AH and HF. However, valsartan was better tolerated due to the significantly lower incidence of cough in Azerbaijan patients. Men did not reveal significant differences in efficiency of starting therapy with valsartan and perindopril. Differences in drug pharmacokinetics in women and men may be due to lower body mass with smaller organs dimension and higher amount of adipose tissue in women. Distinctions in hormone level in men and women can impact on drugs absorption and elimination [17]. Sex-related differences in enzymes of cytochrome P450 system activity were also found [18].
In Europe study perindopril was effective in cardiovascular prevention in high risk male patients but its efficacy was not valid in female patients. These distinctions can be explained by specific properties of some drugs or by RAAS activity level in the study groups [19].
Analysis of many clinical trials which studied ACEi demonstrated their significantly lower efficacy in women than this in men. This is particularly attributes to ACEi impact on CHF clinical outcomes [20]. There is no answer why women with CHF have lower mortality when taking ARB, rather than ACEi, ARB and ACEi are used for BP control and for lowering of neurohormonal activity increased in CHF patients. Medicines of the both groups inhibit angiotensin effects or production by different ways [14]. Whats are the reasons for such results? First of all they may be due to particularities of ACE functioning in female organism. On the second hand, it can be the result of specific samples formation in the trials. The total share of women in these studies was known to be not large. Despite of large-scale study results (LIFE,VALUE, ELITE, ValHeFT, VALIANT,OPTIMAAL,CHARM) there is no clearness in the question of sex-related differences in ARB effects. One of explanations is a small number of clinical cases that do not allow realization of meta-analysis [20].
Specific data of biochemical distinctions in sensitivity to RAAS inhibitors are absent for the present, and further researches are needed.
Conclusion
Angiotensin II receptor blocker (valsartan) turned out to be more effective as a start therapy in women than perindopril. Men did reveal significant distinctions in the efficacy of the start therapy with perindopril and valsartan. However, perindopril has a significantly higher incidence of dry cough, leading to discontinuation of treatment in some patients.
Acknowledgement
We are grateful to our respective hospital staff for their assistance in carrying out this study.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosures
Author declare that there is no conflict of interest.
Şəkillər

Açar sözlər
İstinadlar
1. Bui AL; Horwich, TB; Fonarow, GC (January 2011). "Epidemiology and risk profile of heart failure.".Nature Reviews Cardiology8 (1): 30–41.
2. Mann DL, Chakinala M (2012). Harrison's principles of internal medicine: Chapter 234. Heart Failure and Cor Pulmonale. (18th ed.). New York: McGraw-Hill. ISBN 978-0071748896.
3. Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. HCUP Statistical Brief #160. Agency for Healthcare Research and Quality,
4. Rockville, MD. August Rathore SS, Foody JM, Wang Y, Herrin J, Masoudi FA, et al.: Sex,quality of care, and outcomes of elderly patients hospitalized with heart failure: Findings from the National Heart Failure Project. AmHeart J2005;149:121 – 8.
5. Simpson CR, Hannaford PC, Williams D: Evidence for inequalities in the management of coronary heart disease in Scotland. Heart2005;91: 630-4.
6. Clinical Quality Improvement Network Investigators. Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy. Arch Intern Med 1996;156: 1669-73.
7. Mejhert M, Holmgren J, Wandell P, Persson H, Edner M: Diagnostic tests, treatment, and follow-up in heart failure patients-is there a gender bias in the coherence to guidelines?Eur J Heart Fail 1999;1:407-10.
8. Haldeman GA, Croft JB, Giles WH,Rashidee A: Hospitalisation of patients with heart failure: national hospital discharge survey, 1985 to 1996.Am Heart J 1999;137:352- 60.
9. Philbin EF, DiSalvo TG: Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am J Cardiol1998;82:76 – 81.
Harjai KJ, Nunez EJ, Humphrey S, Turgut T, Shah M, et al.: Does gender bias exist in the medical management of heart failure? Int J Cardiol 2000;75: 65-9.
10. Stromberg A, Martensson J: Gender differences in patients with heart failure. Eur J Cardiovasc Nurs2003;2:7-18.
11. Vaccarino V, Chen YT, Wang Y, Radford MJ, Krumholz HM: Sex differences in the clinical care and outcomes of congestive heart failure in the elderly. Am Heart J 1999;138: 835-42.
12. Pearson ML, Kahn KL, Harrison ER, Rubenstein LV, Rogers WH, et al.: Differences in quality of care for hospitalized elderly men and women. JAMA 1992;268:1883- 89.
13. Altimir S, Lupon J, Gonzalez B, Prats M, Parajon T, et al.: Sex and age differences in fragility in a heart failure population. Eur J Heart Fail 2005;7:798 – 802.
14. Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensinconverting enzyme inhibitors in
patients with congestive heart failure; A population study. Heart Fail. 2007; 9:602-9.
15. Weinshilboum R. Inheritance and Drug Response. N Engl Med 2003; 348(6): 529-37.
16. Evans WE, McLeod HL. Pharmacogenomics- Drug Disposition, Drug Targets, and Side Effects. N Engl] Med 2003; 348(6): 538-49.
17. Prokhorovich E.A., Tkacheva O.N, Adamenko A.N. Clinical course and treatment of hypertensive women.Trudniy Pasient 2006;13-7.(Russian).
18. Contreau M.M., von Moltike L.L.,Greenblatt D.J. The influence of age and sex on the clearance of cytochrome P4503A substrates. Clin Pharmacokinet 2005;44:33-60.
19. Ivleva A.Ya. The study of the effectiveness of cardiovascular drugs in women.Kardiologia 2006;3:85-9 (Russian).
20. Tereshenko S.N., Zhirov I.V. Gender differnces in chronic heart failure ;myth or reality. Problemy Zhenskogo Zdorovya 2007;1 (2):69-4.
Məqalə barədə təfərrüatlar:
Nəşr tarixçəsi
Dərc edilib: 20.Jul.2015
Müəllif hüququ
© 2013-2025. Azərbaycan Kardiologiya Cəmiyyətinin rəsmi nəşri. Jurnal "Uptodate in Medicine" tibb nəşriyyatı tərəfindən dərc olunur. Bütün hüquqlar qorunur.Əlaqəli məqalələr
Baxılıb: 816


